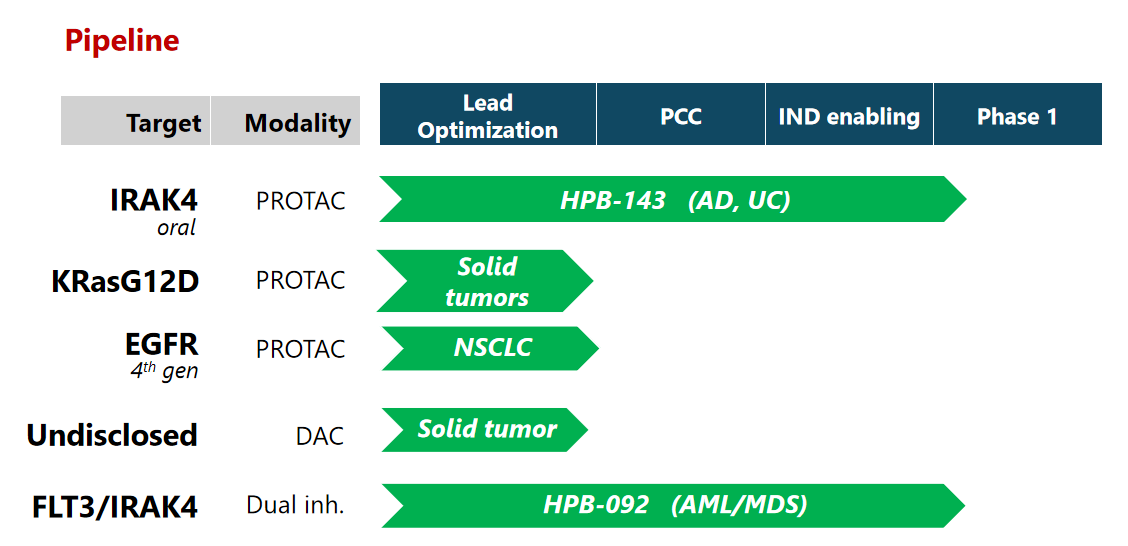
HPB-143 (oral IRAK4 PROTAC)
In the Myd88/IL-1R signal transduction pathway, IRAK4 serves dual functions as a kinase and a scaffold protein in regulating the inflammatory responses. HPB-143 has received IND approval in both the US and China.
KRasG12D PROTAC
KRasG12D is an oncogenic driver most prevalent in pancreatic and colorectal cancer. A PROTAC KRasG12D degrader is expected to completely shut down the downstream MAPK and PI3K pathways. The program is on track to enter the clinic in 2026Q2.
EGFR PROTAC
Oncogenic driver mutations in EGFR is highly prevalent in non-small cell lung carcinoma. Moreover, drug-resistance mutations often arise in the same EGFR gene (T790M and C757S) that result in resistance to the stand-of-care first line EGFR inhibitor Tagrisso. A PROTAC against EGFR covers all these mutations and provides an effective therapeutic approach to combat the resistance. The program is on track to enter the clinic in 2026Q3.
Degrader Antibody Conjugate (DAC)
Conjugating a PROTAC molecule with a targeting-antibody is an emerging modality that can improve selectivity and pharmacokinetic property of the drug. We are exploring a number of combinations for the treatment of solid tumors. The most advanced program is on track to enter the clinic in 2027Q1.
HPB-092 (FLT3/IRAK4 dual kinase inhibitor)
FLT3 oncogenic mutations occur in 25-30% AML patients. While FLT3 inhibitors have been approved for AML patients in the clinic, treatment-induced resistance mediated by IRAK4 presents unmet medical need. The FLT3/IRAK4 dual inhibitor HPB-092 is expected to have deeper and more prolonged response than the stand-of-care FLT3 inhibitors. HPB-092 has received IND clearance in both China and the US.
